The fight against neurodegenerative diseases and in particular against Alzheimer's disease is the first "priority axis" on which the Foundation for Medical Research positions itself in its ambition to increase the impact of its action, i.e. to promote the best biomedical research. at the service of patients. It is in this context that we have decided to carry out a whole process of collecting information and achievements in the sector, with an international outlook. This newsletter serves as an introduction to this dynamic and we hope that it will please your teams and you.
Good reading !
Clinical studies: where are we? What is the outlook for Alzheimer's disease?
We've gathered the most up-to-date key data about ongoing clinical trials tackling Alzheimer's disease.
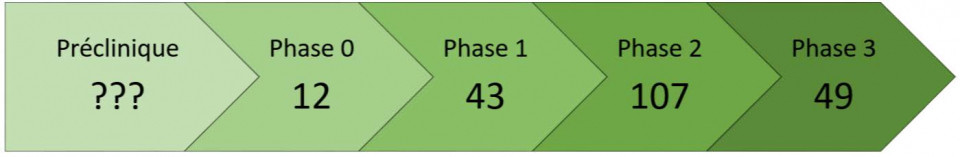
Difficult to know the real number beyond these figures which already show the significant enthusiasm around this line of research. The aging of populations, the societal and economic impact of the disease are probably among the key issues to be taken into consideration.
There is room for other initiatives! Unfortunately, it is impossible to have the figures on the preclinical studies, which are not published...
For those who wish, a little reminder on the progress of clinical trials.
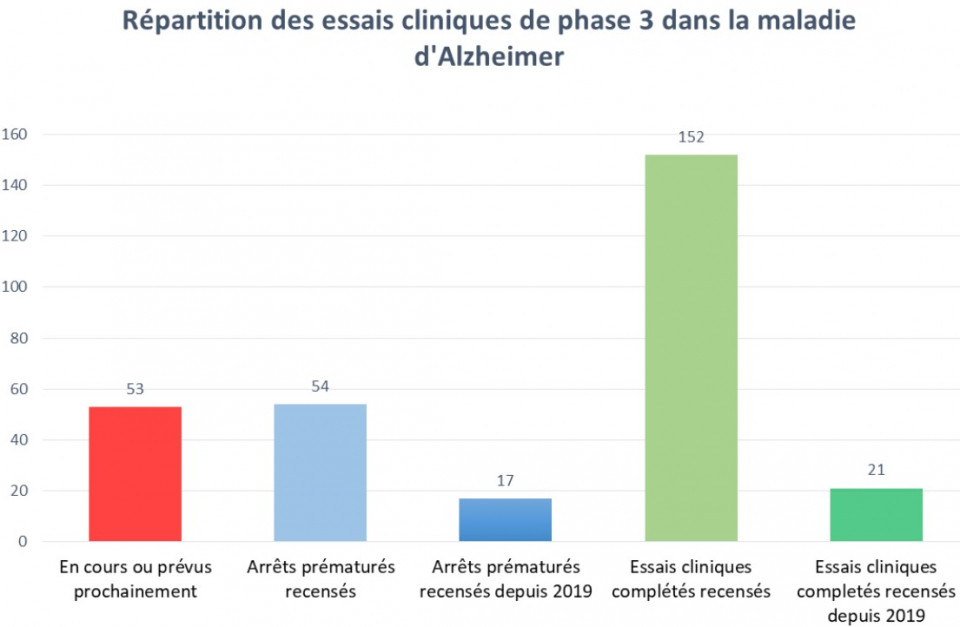
Of the 200 clinical trials now completed, a quarter have been terminated prematurely, a good proportion of which since 2019. This figure does not show all phase 1 and 2 clinical trials that have failed. But worse, one hundred and fifty-two completed phase 3 clinical trials have allowed only four compounds to obtain marketing authorization. And again, these were delisted in August 2018 for lack of effectiveness. Despite this, the search for treatments for Alzheimer's disease remains very active with more than 50 current clinical trials.
Who says clinical study says financing of clinical studies…. Who are the main actors?
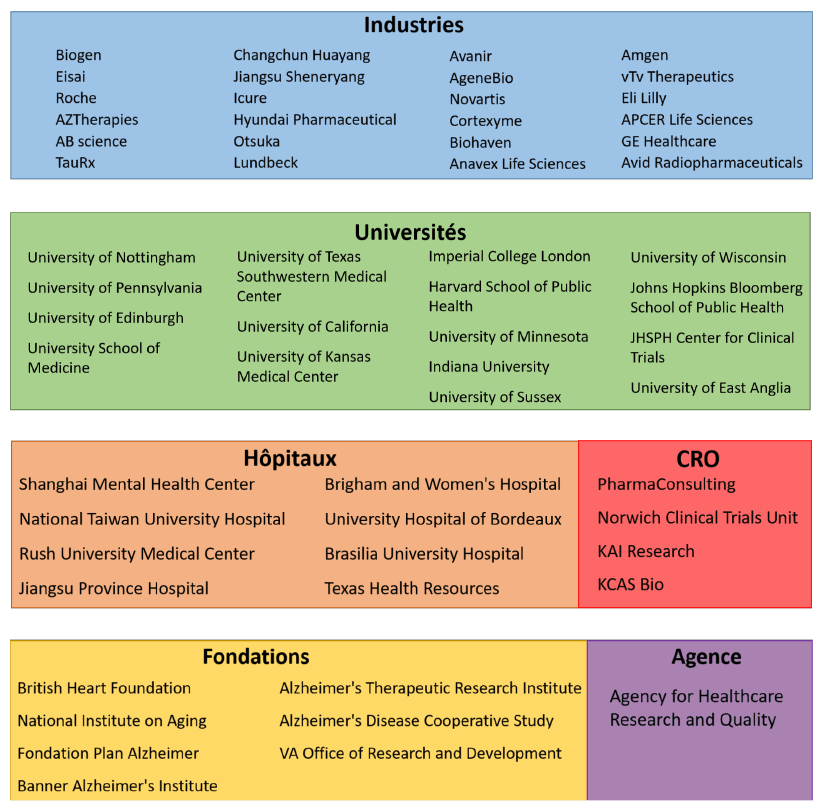 List of sponsors of current Phase 3 clinical trials in Alzheimer's disease listed on clinicaltrials.gov for clinical studies available on June 15, 2020.
List of sponsors of current Phase 3 clinical trials in Alzheimer's disease listed on clinicaltrials.gov for clinical studies available on June 15, 2020.
The main sponsors are therefore pharmaceutical industries, universities, hospitals, particularly in China, foundations (not yet the FRM!), contract research companies and an American federal agency.
First selection of already advanced US companies and young French shoots Beyond these clinical aspects and the few names presented above, advancing research against Alzheimer's disease requires scientific bases of excellence, development capacities, of energy and to integrate the competitive as well as the global competitive environment.
Here is a first overview with some selected start-ups:
Alzheon is an American company founded in 2013 by Martin Tolar. It is currently developing a tramiprosate prodrug, ALZ-801. Tramiprosate is a drug studied for 20 years, having been the subject of a phase 3 clinical trial in 2004. Despite the failure to obtain the Marketing Authorization, an analysis of the clinical trials carried out has shown very encouraging results on a sub-population, homozygous APOE4/A4. Alzheon decided to develop a compound similar to tramiprosate by targeting only APOE4/4 homozygotes and using a reworked dosage form. A phase 3 clinical trial of ALZ-801 is planned soon. It will last 18 months and will use the ADAS-Cog11 scale as the primary endpoint.
AgenT was created in 2018 by two Frenchmen, Jérôme Braudeau and Baptiste Billoir. It aims to develop a medical device to diagnose Alzheimer's disease in the blood. The technology aims to detect Alzheimer's disease ten to twenty years earlier than the current diagnosis, as well as to stratify the progression of the disease. It was created in an innovative model of rats suffering from slowly progressive Alzheimer's disease, and uses more than one hundred blood biomarkers to predict the state of the brain. It is currently being tested on five hundred blood samples from patients, some of which were taken twenty years ago, to be validated in a controlled clinical study next year in order to initiate marketing after 2022.
Michela Gallagher founded the biotechnology company Agenebio in Baltimore in 2014, currently carrying out a phase 3 trial involving 830 patients (HOPE4MCI study), summarized on this poster. The molecule tested is levetiracetam at doses twelve times lower than those used when prescribing it as an antiepileptic. The company's promise is to decrease hippocampal hyperactivity and limit memory loss. The HOPE4MCI study uses the CDR-SB (Clinical Dementia Rating – Sum of boxes) score as the primary endpoint following 18 months of treatment versus placebo.
NeoNeuro is a French start-up created in 2015, resulting from a collaboration between a Canadian company, NeoVentures biotechnology and the Institut du Cerveau et de la Moelle, led in particular by Professors Dubois, Duyckaerts and Hampel as well as Doctor Potier. Its objective is to predict the state of the patient's brain based on a blood test. To do this, Neoneuro has developed a platform to screen all the epitopes present in the blood using aptamers, i.e. DNA or RNA molecules capable of recognizing ligands or catalyzing reactions. chemical or biochemical. Two cohort studies are underway in France and Australia to validate the method.
Who are the giants and major players in the sector?
We have chosen 3 companies active in the fight against neurodegenerative diseases. In addition to their significant capacities, they can also be a source of partnerships... or additional funding!
Lundbeck is a Danish pharmaceutical company specializing in the development of drugs in neurology and psychiatry. It employs 5,000 people and achieved a turnover of 2.4 billion euros in 2018. Lundbeck currently markets a dozen drugs including memantine and is conducting phase 3 clinical trials on a compound against evening restlessness in Alzheimer's disease, brexpiprazole. This partial D2 agonist antipsychotic is already used in schizophrenia and major depressive disorder.
Biogen is an American pharmaceutical company specializing in neuroscience employing 7400 employees, with a turnover of 14.4 billion euros in 2019. Regarding Alzheimer's, Biogen currently has five candidates in its pipeline. Two antibodies directed against amyloid plaques are in phase 3, an anti-tau protein antibody is in phase 2 and a second in phase 1, as well as an antisense oligonucleotide that slows down the accumulation of tau protein.
Roche is a pharmaceutical laboratory specializing in biotechnology and innovative therapies, the leading investor in R&D in the health sector. Roche mainly fights against diseases of the nervous system, cancers, hemophilia and respiratory diseases. This company of 94,000 employees achieved a turnover of one billion euros in 2018. 4 anti-Alzheimer drugs are in development: one in phase 3 (anti-Abeta antibody), two in phase 2 (anti-Abeta antibody). -Abeta and anti-Tau antibodies) and one in phase 1 (antibody studied in phase 3 + “brain shuttle” system to improve the crossing of the blood-brain barrier).
Want to know more ?
This article published on January 6, 2020 analyzes ongoing clinical trials and the main causes of failure of recent clinical trials. In particular, there is a typology of the therapeutic targets targeted in recent years and details on the substances currently undergoing clinical trials.
We hope you enjoyed it and look forward to your feedback!

Comments0
Please log in to see or add a comment
Suggested Articles