Following the success of the first newsletter and your feedback (thank you!), we are happy to present the second Alzheimer newsletter! On the program: the very latest news on research into Alzheimer's disease, an analysis of a phase 3 clinical trial and feedback on the promotion of research projects.
Good reading !
Two advances in the search for a cure
The beginning of July 2020 was loaded with announcements in the fight against Alzheimer's disease: a request for marketing authorization in the United States and the launch of a phase 3 clinical trial.
- On July 8, 2020, Biogen and its partner Eisai announced that they had filed a marketing authorization application with the Food and Drug Administration. It concerns aducanumab, an antibody directed against aggregated amyloid plaques, tested in humans since 2012. This news is all the more surprising as it comes after the early interruption of phase 3 clinical trials (the story details can be found below).
- These same two companies have started a phase 3 clinical trial in the United States to test the effectiveness of their BAN2401 antibody targeting beta-amyloid protofibrils. The first clinical trial carried out on this molecule is now ten years old.
We note that Europe is currently behind!
Clinical trials of aducanumab, failure or success?
In 2015, Biogen started two parallel phase 3 clinical trials, EMERGE and ENGAGE, which were expected to enroll just over 1,600 patients each. The design of the studies was similar, namely monthly administrations of low or high dose aducanumab or of a placebo for seventy-eight weeks. The primary endpoint was the level of cognitive decline and patient autonomy, measured by the Clinical Dementia Rating - Sum of Boxes scale. These studies are stopped early on March 21, 2019 because an interim analysis carried out on 1748 participants predicts that the main objectives have statistically very little chance of being achieved. However, data continues to be collected and a new analysis is carried out on the initially planned sample of more than 3200 patients. This concludes that high-dose aducanumab was significantly effective in the EMERGE study, but not in the ENGAGE study.
What happened ?
The main explanation lies in the design of the study and in particular the protocol changes. Indeed, in APOEε4+ patients, treatment at the maximum dose of 10mg/kg could cause cerebral edema. However, the associated clinical symptoms are not very disabling (headaches, dizziness, nausea) and the edema disappears spontaneously within four months. This is why APOEε4+ patients included in the study before the fourth version of the protocol received a dose of 6 mg/kg (in purple on the graph below) against 10 mg/kg for those included after the new protocol (in green on the graph below).
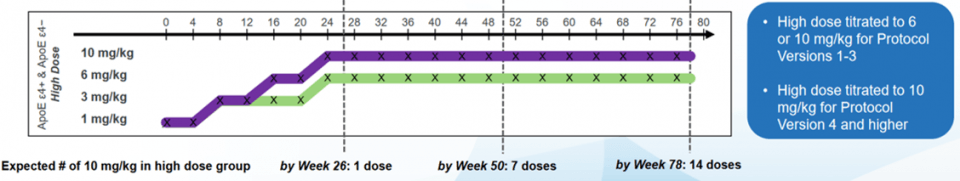
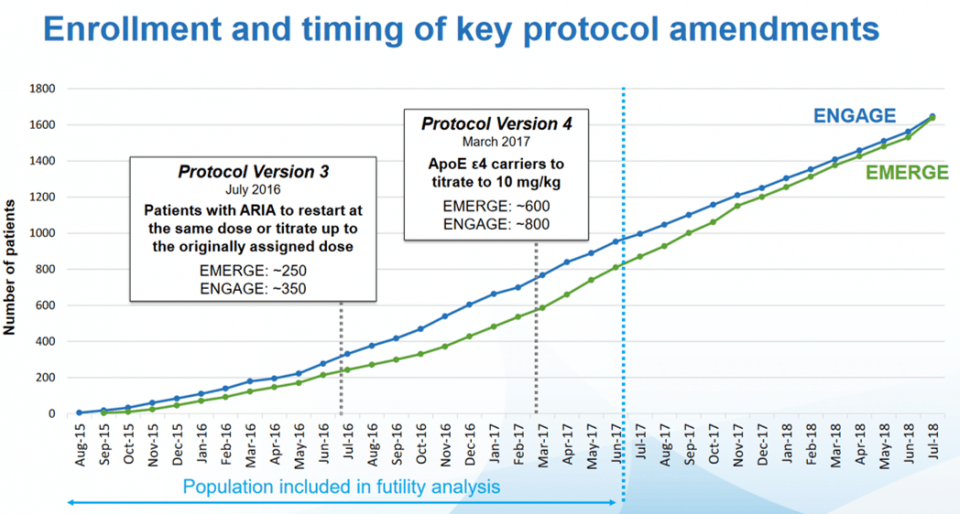
On the other hand, the ENGAGE study started one month earlier than the EMERGE study and included more APOEε4+ patients treated with the old protocol which did not allow the dose of 6mg/kg to be exceeded. This resulted in lower mean aducanumab doses than in the ENGAGE trial. Indeed, 22% of ENGAGE patients received the 14 injections at 10 mg/kg compared with 29% of patients in the EMERGE study. This explains the best results of the second clinical trial.
This graph shows the number of patients included in each study over time. It is interesting to note that the majority of the data used in the interim analysis come from patients treated according to the old protocol.
In the subgroup treated with the fourth version of the protocol in the ENGAGE study, there is a trend of improvement in the Clinical Dementia Rating - Sum of Boxes scale in the treated patients, but this is not statistically significant. Despite these encouraging results, which show in particular that a strategy targeting beta-amyloid peptides can be effective in certain patients, there are still many gray areas to be clarified. It is for this reason that Biogen plans, in parallel with the Marketing Authorization application, to carry out a new clinical trial using the dose of 10 mg/kg.
In conclusion, this clinical trial underlines the importance of the methodology and the clinical question asked coupled with the anticipated statistical analysis. It is essential to select a patient population that is not too heterogeneous and to define a precise clinical question so that the study can be interpreted.
The full results of these clinical trials are available in this slideshow from Biogen.
After an overview of the advanced projects, look back to the future with the perspectives of valorization and how to anticipate the challenges as early as possible in the life of an academic project.
Interview with a researcher turned entrepreneur, Revital Rattenbach.
How to anticipate valuation from the start of the project? Interview with an expert on the subject

Can you briefly introduce yourself and your background?
I am the founder and president of the company 4P-Pharma whose ambition and objective is to develop real treatments for diseases related to aging based on the discoveries of academic researchers. I had an academic career with a thesis in biology of aging during which I became interested in the link between trisomy 21 and the cellular mechanisms of aging and in particular Alzheimer's disease. After a post-doc, I had the chance to be responsible for the promotion of academic research within a European consortium. This is how I was able to co-found my first company, a spin-off from Sorbonne University, and discover the world of entrepreneurship.
What are the specificities to be taken into account for valorization in the field of Alzheimer's disease?
Alzheimer's disease, like most diseases related to aging, is multifactorial in terms of risk factors, clinical signs or symptoms. There is no clear correlation between MRI imaging or liquid biomarkers and patient symptoms. The population is heterogeneous. The best “treatment” today being cognitive exercises and the patient’s entourage.
It will therefore be very difficult to assess the positive effects of a treatment on the progression of the disease. These limitations must be taken into account from the start of the development of a treatment by trying to analyze very specifically what are the mechanisms targeted by the candidate drug and trying in an optimal way to stratify the population by trying to predict the best responders to the mechanism. of action whether at the stage of the disease, at the level of liquid biomarkers or common imaging.
In your opinion, what are the avenues of reflection to be favored in order to target this disease?
Academic research in Alzheimer's disease is very rich and yet no lead has provided solutions to patients. The number of failures in clinical trials is gigantic. Several reviews that bring together the leads favored until today show that more than 70% of the treatments tested in the clinic target the mechanisms linked to ab or tau. In recent years with the development of biomarkers, although they are not yet validated, research has targeted the early mechanisms of the disease such as inflammation, ab clearance mechanisms, risk factors such as depression or Metabolic syndrome again.
Do you have any key messages to convey to researchers regarding the promotion of their research projects?
When an academic researcher discovers a therapeutic avenue, he must not hesitate to promote it, drugs almost always originate from this university and/or hospital research. However, academic research and drug development are very different professions and require different expertise and experience. The researcher is essential for the discoveries and the knowledge he has acquired by working on the disease or on the target he favors and will always be essential to the development of the drug. To improve the chances of success, the researcher must therefore be advised by pharmaceutical development experts.
We hope you enjoyed this second newsletter!

Comments0
Please log in to see or add a comment
Suggested Articles


