Alzheimer Newsletter #5 - Neurodegenerative diseases
We are pleased to present to you the fifth newsletter of the Foundation for Medical Research on Neurodegenerative Diseases.
This newsletter is primarily intended for the awardees of the "first priority axis" of the FRM, i.e. the awardees of the 2019 Alzheimer and 2020 Neurodegenerative Diseases calls for projects - but do not hesitate to share it if you wish. . The first three newsletters focusing on Alzheimer's disease were only sent to the winners of the 2019 calls, do not hesitate, for those who have not received them, to contact us if you wish to receive them.
On the program of this newsletter:
![]() recent publications by winning researchers from the “first priority area”
recent publications by winning researchers from the “first priority area”
![]() the latest results of phase 3 clinical trials in the field of neurodegenerative diseases as well as those expected by the end of the year
the latest results of phase 3 clinical trials in the field of neurodegenerative diseases as well as those expected by the end of the year
![]() the changes brought about by the PACTE law in the status of the researcher-entrepreneur
the changes brought about by the PACTE law in the status of the researcher-entrepreneur
![]() research valorization webinars scheduled for September 2021
research valorization webinars scheduled for September 2021
Have a good reading!
News within the “first FRM priority area” network
Focus on 2 articles recently published by winners of the "first priority axis". Congratulations to them for these publications!
Chronic Sodium Selenate Treatment Restores Deficits in Cognition and Synaptic Plasticity in a Murine Model of Tauopathy
![]() Ahmed et al.; Front Mol Neurosci. 2020 Oct 8 doi: 10.3389/fnmol.2020.570223.
Ahmed et al.; Front Mol Neurosci. 2020 Oct 8 doi: 10.3389/fnmol.2020.570223.
This study shows that a 3-month treatment with sodium selenate, an activator of protein phosphate 2A (PP2A), in a transgenic mouse model of tauopathy restores cognitive functions
In addition, this treatment reduces the activity of the catalytic subunit of PP2A without affecting total PP2A. This study reinforces the idea that the negative impact of Tau pathology on plasticity and memory is likely due to non-aggregated soluble Tau species. Sodium selenate could therefore be a candidate for clinical trials targeting Tau pathology.
Atomic Force Microscopy Imaging and Nanomechanical Properties of Six Tau Isoform Assemblies![]() Maki et al. ; Biophys J. 2020 Dec 15; doi: 10.1016/j.bpj.2020.10.045.
Maki et al. ; Biophys J. 2020 Dec 15; doi: 10.1016/j.bpj.2020.10.045.
In this study, Tau amyloid fibrils were generated from 6 Tau protein isoforms. Regardless of the isoform, the fibrils exhibit a structure of paired helical filaments. The N-terminal inserts do not contribute to morphological or mechanical differences between the filamentous forms of the Tau isoforms, whereas the fibrils made up of the isoforms comprising 4 microtubule-binding domains are twice as rigid as those comprising 3. low values of moduli of elasticity suggest that the bonds between Tau molecules in a pleated beta form in the filaments and which play an important role in the stability of the filaments but also in their mechanisms of toxicity and propagation are weak. Additional studies are still needed to document this last aspect.
What about clinical trials on neurodegenerative diseases?
You are probably wondering if the results of phase 3 clinical trials announced in NL#3 for the end of 2020 - beginning of 2021 have indeed been announced. Here is a selection of the main results published since November 2020 and the results expected by 2022.
- June 7, 2021: Conditional FDA approval of aducanumab (Biogen)
The FDA has announced "fast track" approval of aducanumab, a treatment for Alzheimer's disease, and is calling for a 9-year post-approval trial to verify the drug's clinical benefits. Aducanumab is an anti-amyloid monoclonal antibody that reduces amyloid load in the brain. Due to the history of this clinical study which had been prematurely interrupted in 2019 and for which the FDA's commission of independent experts issued a negative opinion in November, the decision taken by the FDA was not unanimous in the scientific community. Following these controversies, the FDA announced on July 7, 2021 that it had modified the recommendations for use of the drug, which is now indicated for "patients with moderate cognitive impairment or in a moderate dementia phase of the disease". Aducanumab is only authorized in the United States, the EMA (European Medicines Agency) will issue its opinion by the end of 2021.
Sources : FDA : Aducanumab Information ; France Alzheimer : Focus sur l’aducanumab ; FCM : Aducanumab : épisode VII, l’approbation par la FDA ; The Economist : America’s wary approval of an Alzheimer’s drug offers hope to millions ; Le Quotidien du Médecin : la FDA restreint l'aducanumab aux cas les moins avancés
- April 5, 2021: Rejection by the FDA (US Food & Drug Administration) of the extension of indication for pimavanserin (ACADIA)
The FDA has rejected Acadia Pharmaceuticals' application for approval of Nuplazid (pimavanserin) as a treatment for delusions and hallucinations in patients with broad dementia. Acadia Pharmaceuticals believes this decision contradicts previous feedback from the FDA and disagrees with the FDA's approach to evaluating patients with dementia.
- January 18, 2021: publication of the first results of the phase 2/3 clinical trial of troriluzole (Biohaven)
Biohaven Pharmaceutical has published an outline of the results of the phase 2/3 clinical trial of troriluzole as a symptomatic treatment for mild to moderate forms of Alzheimer's disease. The treatment works no better than a placebo in measures of cognition and dementia at 48 weeks. The full results of this study are expected in the coming months.
- November 18, 2020: last patient visit of the phase 3 clinical trial of ALZT-OP1 (AZTherapies)
AZTherapies announced that the final patient visit of the Phase 3 COGNITIVE clinical trial evaluating the safety and efficacy of ALZT-OP1, a treatment for patients with early-stage Alzheimer's disease has been completed and data analysis would soon begin. ALZT-OP1 is a combination of 2 molecules already marketed in other indications which aims to put microglia in a neuroprotective state, thus reducing neuroinflammation.
And what are the expected results for the next few months?
- September 2021: results of the phase 3 clinical trial of Valbenazine (Neurocrine Biosciences)
Valbenazine is an FDA-approved VMAT2 inhibitor for the treatment of patients with tardive dyskinesia. This clinical trial will test its effectiveness on chorea associated with Huntington's disease.
- September 2021: results of the phase 3 clinical trial of oxycodone PR and levodopa (CHU, Toulouse)
This study evaluates the analgesic effects of oxycodone and levodopa on chronic central neuropathic pain caused by Parkinson's disease. They will be administered in addition to the usual antiparkinsonian treatment.
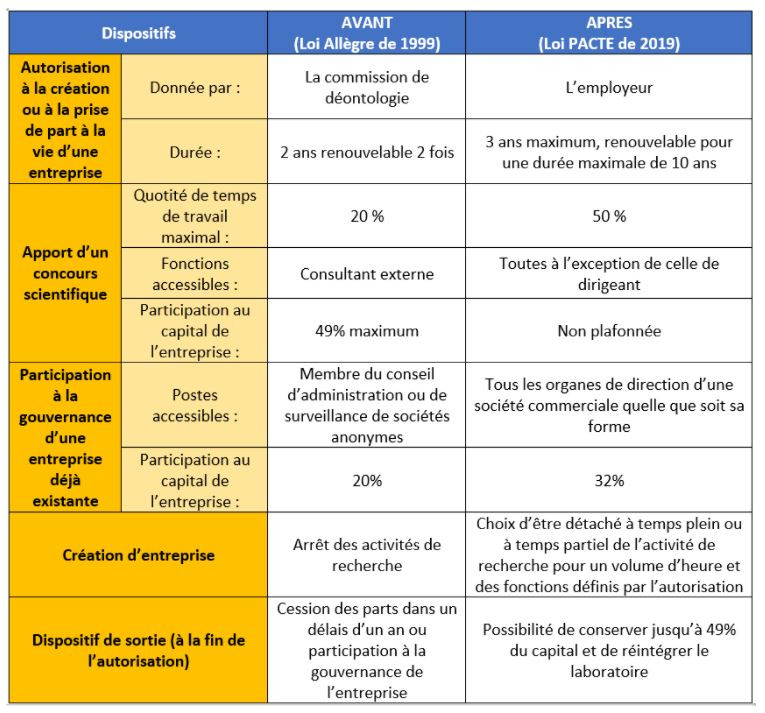
Do you know the PACTE law? Since 2020, it has made life easier for the researcher-entrepreneur
The PACTE law (Action plan for the growth and transformation of companies), adopted in 2019, aims to give companies the necessary means to innovate, transform, grow and create jobs. In the following, we present the main measures of this law concerning the status of researcher-entrepreneur and the protection of intellectual property.
The Allègre law adopted in 1999 aimed at promoting collaboration between public research and companies. However, the 3 main mechanisms provided for by this law (Article 25-1: business creation, Article 25-2: contribution of scientific assistance and Article 25-3: participation in governance) were considered complex and too rigid. Article 41 of the PACTE bill, most of whose measures entered into force in January 2020, aims to simplify procedures, take into account the diversity of existing situations and streamline transitions from one system to another. in order to create more bridges between the world of research and that of business.
The table BEFORE (Allègre law) / AFTER (article 41 of the PACTE law) presents the evolutions of the various mechanisms of the status of the researcher-entrepreneur.
The PACTE law also provides for measures relating to intellectual property (Articles 40 and 42). Here are the main ones:
- The extension of the utility certificate from 6 to 10 years which can be transformed into a patent application.
- The creation of a provisional patent application: the applicant can thus avail himself of a priority date and subsequently detail his claims, within a maximum period of one year. He can also choose to abandon his request at the end of the period if it no longer meets his needs.
- The creation of a patent opposition procedure before the National Institute of Industrial Property (INPI) to strengthen the legal security of the patent and simplify the procedure that can lead to the cancellation of titles.
- The strengthening of the patent examination procedure which will lead the INPI to carry out an in-depth check of the patentability of the invention, in particular the criterion of inventive step and will thus improve confidence in the French patent system.
Sources : Grenoble INP - UGA : Loi sur l’innovation ; Assemblée Nationale : Projet de loi ; Assemblée Nationale : Etude d’impact ; INPI : Loi pacte : La propriété intellectuelle s’adapte aux nouvelles attentes des entreprises
Do you want to know more about the promotion of research?
Here are some Webinars scheduled for September that may interest you!
- September 7, 2021: “Industrial property without borders” by France Innovation
- September 16, 2021: “Overview of aid for business creation” by Bpifrance Création
Photo : contenu gratuit Canva

Comments0
Please log in to see or add a comment
Suggested Articles


